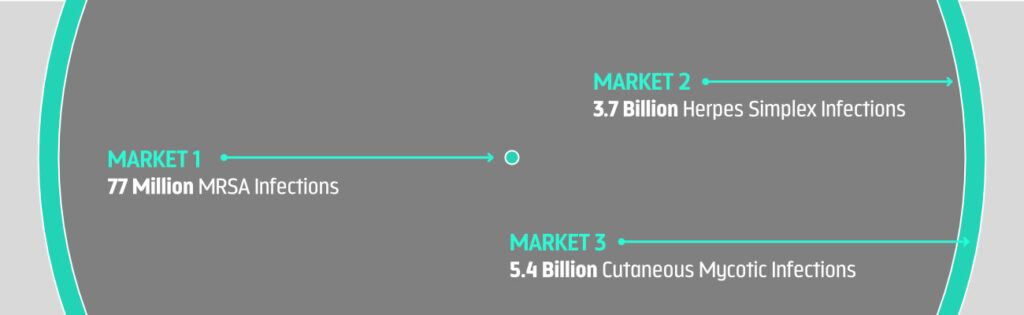
Skin & Soft Tissue Infections Affect Over Half Of The World Population
- 70% of the world population, or 5.4 billion people, experience a cutaneous mycotic infection—a superficial fungal infections of the skin, hair or nails—at least once during in their lifetime
- 67%, or 3.7 billion people under the age of 50, are infected with herpes simplex virus type 1 (HSV-1)
- 1%, or 77 million people, are colonized with MRSA—a multi-drug-resistant staph bacteria
We have assembled an international team of scientists, medical professionals, public health experts, and business partners to collaborate on discovering and developing innovative medicines in the fight against infectious disease.
Our common goal is to improve people’s lives, and advance their health and healing.
MRSA has become resistant to beta-lactam antibiotics, methicillin, aminoglycosides, macrolides, tetracycline, chloramphenicol, lincosamides, and disinfectants. MRSA is particularly dangerous for medical professionals in hospitals in other healthcare environments. Colonization means that bacteria are present but do not currently causing infection. Close contact with MRSA-infected people or animals however, carries the risk of transmitting the bacteria. MRSA can be spread through contact with a contaminated wounds or by touching infected skin. Available treatments for MRSA infections include antimicrobial drug therapies, drainage, and general skin disinfection. For many years, healthcare professionals have been voicing the need for a topical treatment with immediate results, capable of killing the bacteria, and supporting a natural healing process.
We are in phase 1 development of a single-dose, topical treatment for skin and soft tissue infections
caused by multidrug-resistant agents such as MRSA.
In collaboration with our research partners, our team of scientists is advancing patent-protected lab technology, well known to our team and meeting some of the highest benchmarks in efficacy against biological and chemical pathogens. Clinical studies are targeted to start within the next 12 months.
For more information contact [email protected] and a member of our team will get in touch with you.